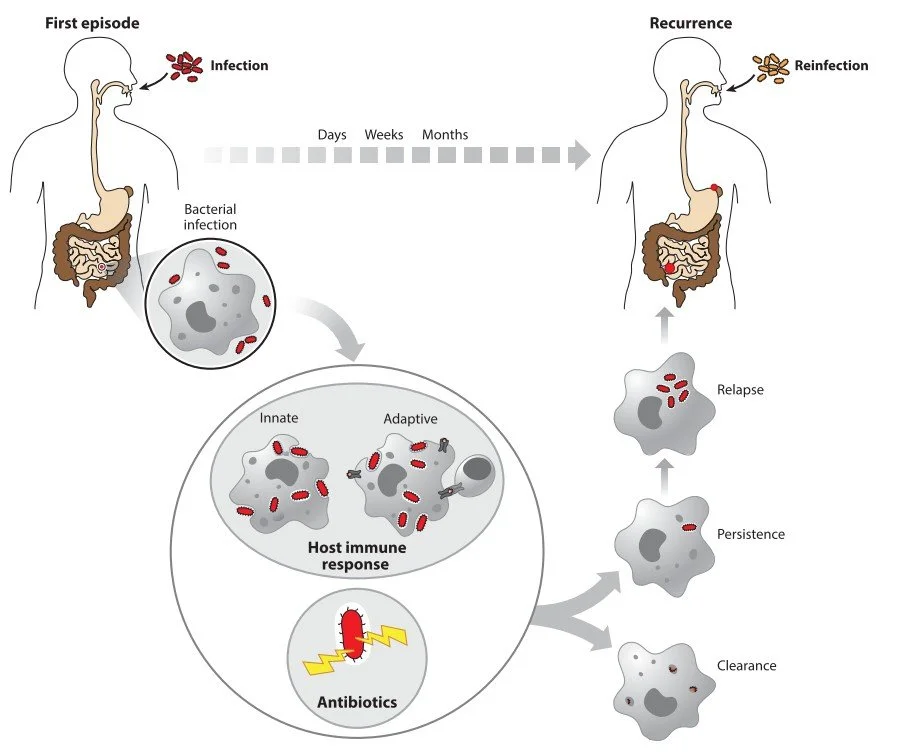
Upon infection, the host initiates a defense through the innate immune response, followed by activation of the adaptive immune system. Key players such as phagocytic immune cells engulf and degrade the invading bacteria. Antigen-presenting cells—including macrophages and dendritic cells—form a critical link between innate and adaptive immunity.
In most cases, the combined action of immune defenses and antibiotic treatment is sufficient to eliminate the infection. However, recurrence can occur. This may result from reinfection (orange) by a new bacterial invasion, or from relapse, where a subpopulation of bacterial persisters survives both antibiotics and immune responses. The yellow lightning bolts symbolize the various stress conditions encountered during infection that can promote persister formation.
From Michaux et al., 2019
Understanding Bacterial Persistence in Infection
Our research focuses on bacterial persisters — a subpopulation of non-growing, transiently antibiotic-tolerant cells that arise within otherwise susceptible bacterial communities. These cells are not genetically resistant, but instead enter a recalcitrant state triggered by environmental stress, enabling them to survive otherwise lethal conditions, including antibiotic treatment.
During infection, pathogens encounter a wide range of host-induced stresses. In response, they can form persisters that are not only tolerant to antibiotics but also evade host immune defenses. These surviving cells are thought to play a key role in chronic and relapsing infections, representing a major challenge in the treatment of infectious diseases.
While the phenomenon of antibiotic persistence has been widely studied in vitro, much less is known about how persisters behave and survive within a host. My work aims to bridge this critical knowledge gap by exploring the interface between antibiotic tolerance and host-pathogen interactions.
Understanding how persisters contribute to infection and treatment failure is essential for developing more effective therapies and combating the growing threat of antibiotic resistance. Through my research, I aim to integrate insights from microbiology, infection biology, and immunology to uncover new strategies for tackling persistent bacterial infections.